Progress Semester 5
Nik Berninger: CNRS Toulouse |
Reaction kinetics of Mg-carbonates. Hydrothermal mixed-flow reactor (HMFR) experiments to investigate magnesite bulk reactivity as a function of aqueous supersaturation and aqueous Ca concentration showed that Ca gets incorporated by up to 8 mol% during magnesite growth. These grown layers were investigated with RAMAN spectroscopy as a function of layer depth. |
Stan Jelavic: Uni Copenhagen |
Month 1: Characterisation of clay fractions from natural rocks. Batch and cryo-XPS measurements of CaCl2 and MgCl2 adsorption on natural illite, IMt1 and chlorite, CCa2. Characterisation and adsorption experiments on natural sandstones and associated clay extractions. Teaching obligations: Geochemistry |
Daniela Meier: Uni Leeds |
I have started to define the table of contents for my PhD thesis. For more info, please click here. |
Giulia Montanari: Uni Copenhagen |
This reporting period was mainly occupied by the manuscript writing process and teaching activities in Geochemistry, Surface Geochemistry and General Chemistry. In addition, I made further experimental studies on calcium carbonate crystallisation in inorganic and organic systems, at different supersaturations. UV-Vis Spectrophotometer measurements, Phreeqc calculations, XRD and SEM analysis were made on liquid and solid samples. |
Cristina Ruiz Agudo: Uni Münster |
3D morphology of barium sulfate particles precipitated in the presence of a commercial copolymer. For more information, please click here. |
|
Biyun Zhen Wu: Maersk Oil and Gas |
This reporting period was mainly occupied by my secondment in Toulouse at CNRS (more information here) and also by an outreach activity I organised during a visit home to Costa Rica. More about this outreach activity can be found here.
|
Fernando Berro Jimenez: West Systems |
An instrument to automatically measure the silica concentration in geothermal waters was finally developed. The instrument was designed for analyzing the yellow-color intensity of silico-molybdate complex generated when amorphous silica contained in water reacts with molybdic acid. This instrument is going to be tested at the Hellisheidi Power Plant (Iceland) during the summer months and the results are hopefully going to be presented during the Goldschmidt conference in Prague. Additionally, the silica precipitation is going to be compared with a refurbished version of Silnuc, (Weres et al, 1980) in order to gain insight into the whole process. Weres et al. (1980) Kinetics of silica polymerization. Report LBL 7033. |
Jan Prikryl: Uni Iceland |
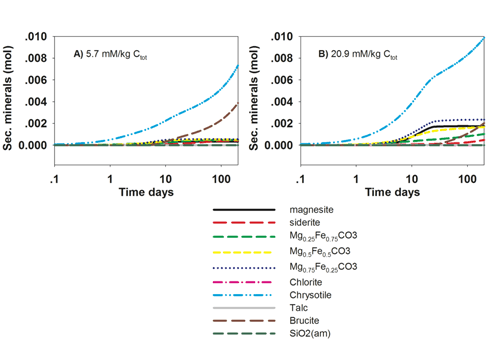
Carbonate Mineral Scaling: For more information, please click here.
|
Diwaker Jha: Uni Copenhagen |
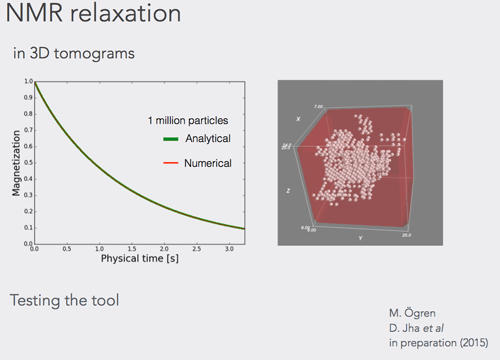
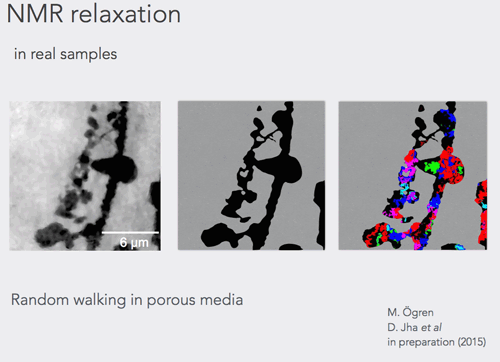
A tool to calculate nuclear magnetic resonance parameters in the porous media geometry obtained from X-ray tomography was developed. The tool can accommodate fast calculation of the NMR properties in the samples up to at least 3 times larger than ever achieved and this in three dimensions.
|
Christopher Hawkins: Uni Oslo |
Month 1: New draft of turbulent mixing paper proposed following data collapse. The turbulent paper is a considerable challenge due to the complex nature of the phenomenon however, a good deal of progress and discovery is achieved during this period and the accuracy of the model confirms the results are reliable – work will continue |
Prathap Moola: Reykjavik Energy |
Gas sampling at Hellisheiði and Nesjavellir geothermal power plant: for more information, please click here. |
|
Taher Rabizadeh: University of Leeds |
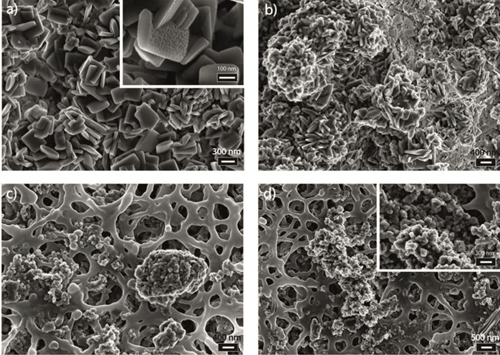
The data that I have produced show that addition of inorganic substances (Li+, Na+, K+ and Mg2+) increased the calcium sulphate induction time. The kinetics of calcium sulphate nucleation and growth was studied by UV-VIS and the solid products were characterized at the end of each reaction of the mineralogical composition by XRD and for their morphological features by SEM. Furthermore, ICP-OES and XPS analyses were utilised to determine the amount of inorganic cations associated with the calcium sulfate crystals; Among monovalent cations, Li+ increase the time needed for turbidity to develop (induction time, start of precipitation) more than the others but a comparison between all of the tested inorganic additives showed that Mg2+ was the best one at delaying the induction time and affecting the crystal growth kinetics. My ICP analysis revealed that by increasing the additive concentrations in the precipitation solution, their association with the precipitated calcium sulfate cyrstal increased; K+ had the highest mount of association which could be because of either incorporation or surface adsorption. The XPS analysis confirmed the presence of K+ and Na+ o or near the surface of the gypsum crystals. These incorporations and surface adsorptions affected the crystals unit cell volume and also changed the morphology of the precipitated calcium sulfate crystals. |