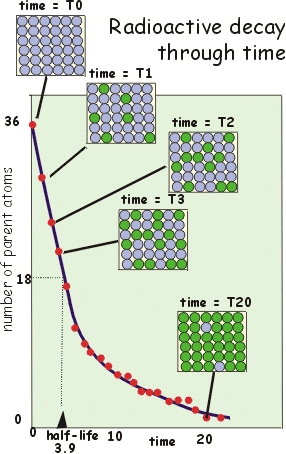

A key principle of radioactive decay is that there is a constant probability per unit of time (e.g. 1 year) of a decay event from parent atom to a new daughter atom. This probability is expressed as the decay constant. Here we explore the consequences graphically. Imagine a batch of 36 parent atoms. These spontaneously decay to daughter atoms (in green). The probability of such a decay for each parent atom is 1/6 per unit of time. So after 1 unit of time the most probable outcome is that 5/6 of the original batch of parents remain (i.e. 30). During the next interval of time 1/6 of the remaining parents will decay (leaving 5/6 of 30 =25 parents). And so it continues. Because the number of parents reduces for each new time interval, the number of events per unit of time reduce (although the probability of each parent decaying is constant). This gives the graph a characteristic shape - exponential decay.
The graph also shows the half-life concept. The half-life is the amount of
time necessary to reduce the number of parent atoms by 50% from the original
number.