Progress Reports Semester 3
Nik Berninger: CNRS Toulouse |
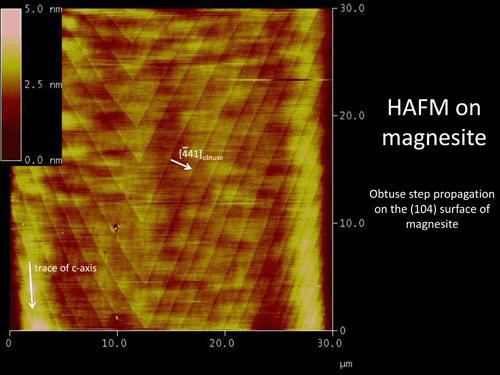
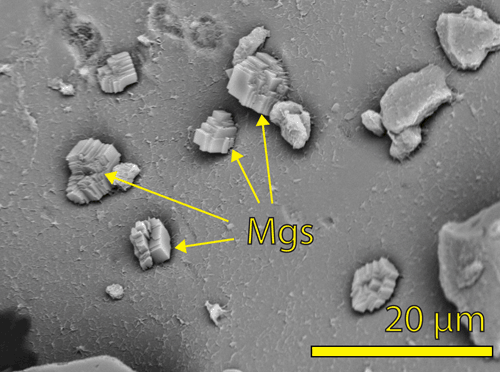
Reaction kinetics of Mg-carbonates. For more information, please click here. |
|
|
Giulia Montanari: University of Copenhagen |
Growth inhibitors on carbonates. For more information, please click here. |
|
|
Cristina Ruiz Agudo: University of Münster |
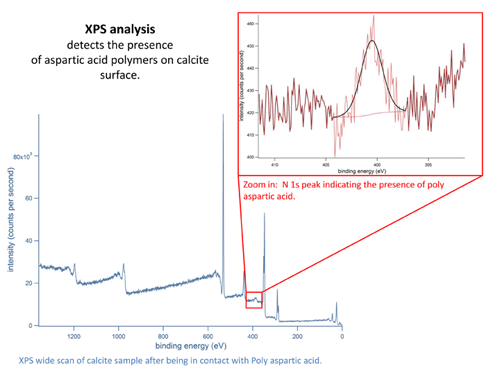
Organic additives as scale inhibitors. For more information, please click here. |
|
|
Daniela Meier: University of Leeds |
Amorphous silica precipitation. For more information, please click here. |
|
|
Jan Prikryl: University of Iceland |
Forsterite+CO2+H2O experimental interactions. For more information, please click here. |
|
|
Fernando Berro Jimenez: West Systems |
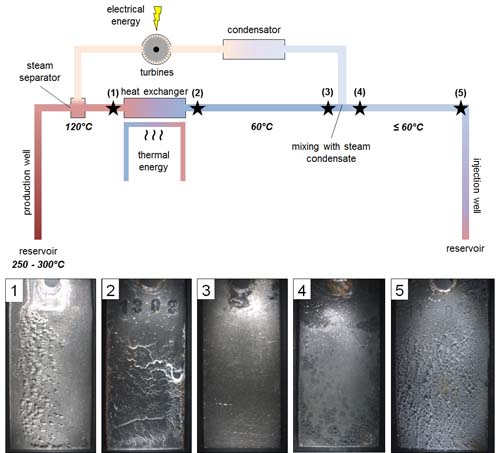
Setting up an automatic device for silica determination on geothermal water |
We are working on a software to manage the instrument for silica colorimetric analysis. We have partially solved the problem of sample dilution by using two pumps (one for geothermal water, the other for the deionized, acidified and SiO2-free water) and a balance. The operation of these instruments is controlled by a software code suitably developed during this study. Next step is to include the reagents for measuring silica and the spectrophotometer as well as to extend the software in order to control the whole process. |
Christopher Hawkins: Uni Oslo |
Month 1: Development of model to simulate turbulent flow using parallel processing An upcoming paper on turbulent mixing in rough channel flow will show the interesting discoveries that have been made in this period To view a presentation given to the American Physical Society in March 2014 in Denver, Colorado, please click here. |
Thomas Rinder: Toulouse |
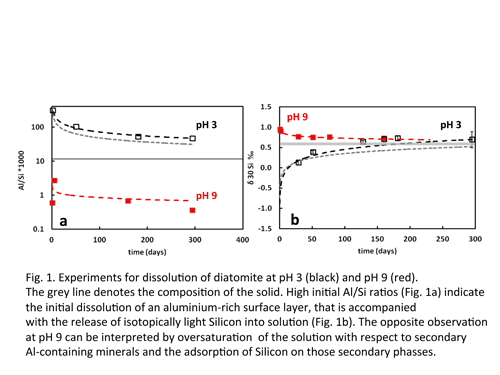
The potential use of Si isotopes as a proxy for monitoring scale formation During CO2 sequestration in Iceland acidic solutions are injected into basaltic rocks, promoting dissolution and shifting the pH up allowing the formation of secondary carbonates. However such a pH increase will also favour the reprecipitation of Fe and Al dissolved from the primary minerals – a process which might negatively influence the rates of mineral carbonation by the formation of passivating Al/Fe-hydroxide coatings on the dissolving phases. The accompanied adsorption of Si on these hydroxides produces a positive shift in the Si isotopic signatures of the remaining solution. Therefore fluid Si isotopic signatures might be applied to monitor injection sites, identify the formation of such detrimental secondary phases and help to take suitable countermeasures. In an attempt to quantify this fractionation process opal-CT, quartz, diatomite, and amorphous SiO2 were dissolved at 70 °C and pH 3 and 9 in closed system batch reactors (I = 0.1) for up to several months, allowing reprecipitation as the solutions reach equilibrium. The isotopic composition of the Si released from quartz is identical within uncertainty to that of the dissolving mineral. In contrast, the Si initially released from opal-CT, diatomite and amorphous SiO2 dissolution at pH 3 is isotopically light but that initially released at pH 9 is isotopically heavy compared with the solid and this fractionation is significantly higher in samples containing aluminium. With time, the Si isotopic composition of the fluid phase at pH 9 converges attaining a stationary state with δ30Si values between 0 to 0.3‰ heavier than the initial solid, as the the dissolved Si concentration approaches equilibrium. The distinct Si fractionation behaviour observed for aluminium containing and pure phases may provide a useful tool for monitoring reprecipitation processes in carbon injection sites. |
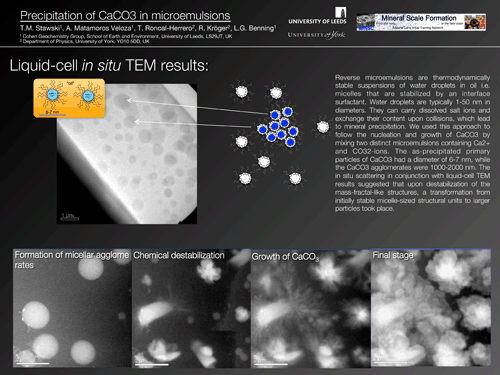
Tomasz Stawski: Leeds |
Essentially the results from the last report are still valid. New results cannot be disclosed publically yet, but we managed to obtain information on destabilization of microemulsions using the liquid cell TEM method. |
Biyun Zhen Wu: Maersk |
Pitzer thermodynamic database from MULTSCALE and PHREEQC geochemical codes were assessed by comparing predicted values with experimental barite solubility data at oil conditions. Barite dissolution experiments at different salinity and oil conditions were conducted in order to test the predicted values. |
Diwaker Jha: Uni Copenhagen |
I had to teach two mandatory MSc courses which once again cut into my research time. However, I finalized the fully functional MATLAB code for "Adaptive ring artifact suppression for tomography applications." |
For more information on adaptive ring artifact suppression for tomography applications, please click here. |
Taher Rabizadeh: Uni Leeds |
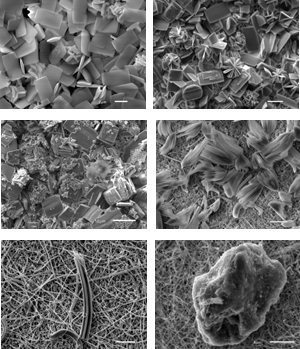
How carbon-chains could help mitigate mineral scaling In several industrial processes that rely on water handling systems (e.g., oil or geothermal energy production, water desalination), the precipitation of mineral scales leads to costly problems including pipeline or membrane clogging. The use of additives to retard mineral formation is a promising approach to mitigate industrial scaling, but the two main requirements for a 'good' inhibitor [1], namely their efficiency at low concentrations and their biodegradability (green inhibitors), are only poorly constrained. we report results from a study on the nucleation and growth kinetics, as well as the structure and morphology of calcium sulfate scale minerals that were precipitated in the presence and absence of low concentrations (0-20 ppm) of 'green' additives of carboxylic and polyepoxysuccinic acids. Nucleation and growth reactions from solution were followed in situ and in a time-resolved manner through changes in turbidity and conductivity, while the solid products were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The results indicate a clear interdependency between additive type and concentration and induction time for the formation of calcium sulfate phases. In the case of carboxylic acids, at equivalent concentrations the most effective inhibition (longer induction time) was achieved with citric acid followed by maleic and tartaric acid [2]. Compared to the additive free system, the presence of 20 ppm of tartaric, maleic and citric acid increased the induction time from 6 min to 9, 16 and 25 minutes. However, by far the most effective inhibitor was polyepoxysuccinic acid, which outperformed all carboxylic acids and at 20 ppm delayed the induction of the reaction to 260 min. This additive clearly inhibited mineral precipitation by at least 40 fold compare to the additive free CaSO4 system. When the solids that caused the turbidity were analysed, the XRD patterns revealed that in all cases at the beginning of the reaction the hemihydrate bassanite (CaSO4. 0.5H2O) precipitated first. With time and depending on additive concentration the hemihydrate bassanite transformed to the dihydrate gypsum (CaSO4. 2H2O) but a clear link between additive type and additive concentrations and bassanite stabilization was evident. Imaging the solid intermediates and end products also demonstrated that some of the additives also changed the shape and size distribution of the solid products, however these effects are still under study. [1] Lioliou et al. (2006), Journal of Colloid and Interface Science 303, 164-170 |
For more information , please click here. |
Prathap Moola: Reykjavik Energy |
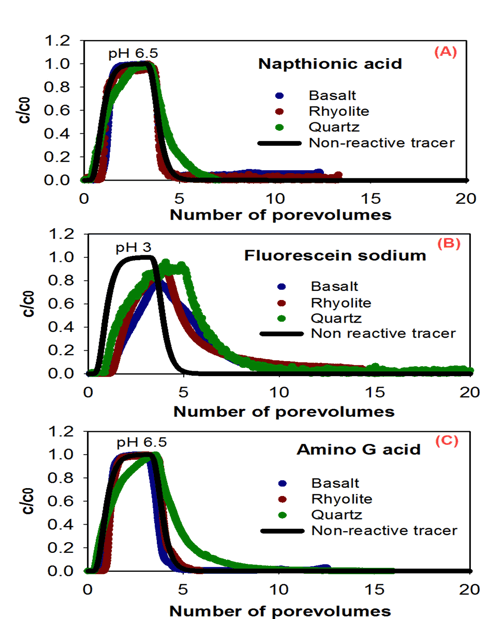
Evaluation of common hydrological tracers in porous rocks. For more information, please click here. |
 |